How Does Chemical Change Affect The Composition Of Matter
Physical and Chemic Backdrop of Matter
- Folio ID
- 237
We are all surrounded by matter on a daily basis. Annihilation that nosotros use, touch, eat, etc. is an case of matter. Matter can exist defined or described as anything that takes up space, and it is equanimous of miniscule particles called atoms. It must display the two backdrop of mass and volume.
Introduction
The dissimilar types of affair can exist distinguished through two components: composition and properties. The composition of matter refers to the different components of matter along with their relative proportions. The backdrop of matter refer to the qualities/attributes that distinguish one sample of matter from another. These properties are generally grouped into two categories: physical or chemical.
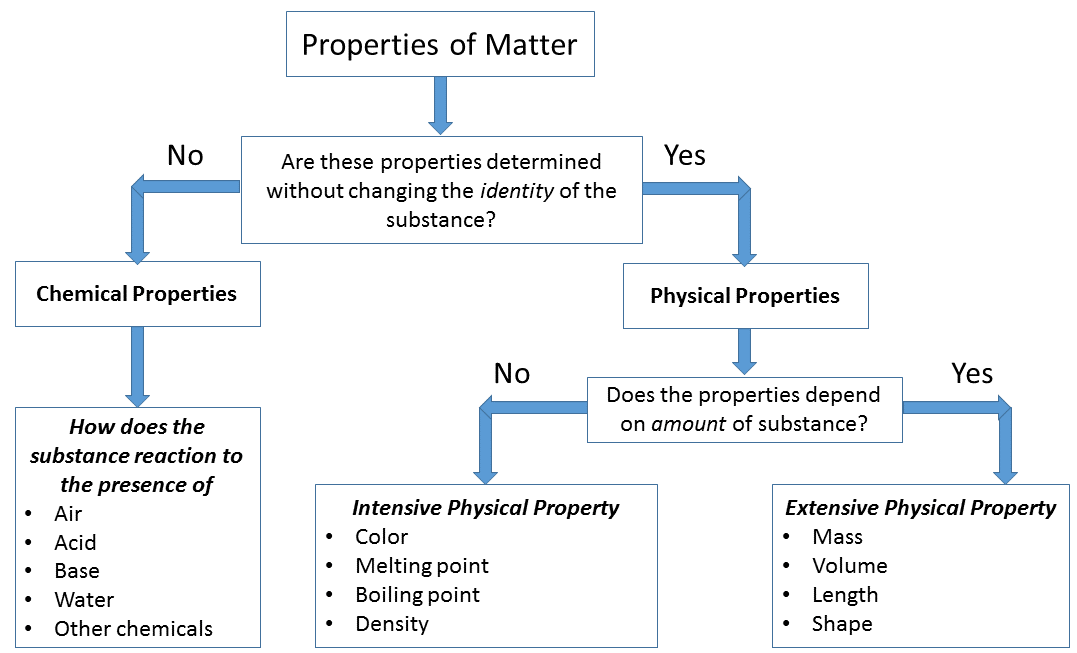
Physical Backdrop and Changes
Physical properties tin can exist observed or measured without irresolute the limerick of affair. Physical properties are used to notice and describe matter. Concrete backdrop of materials and systems are often described as intensive and extensive properties. This classification relates to the dependency of the backdrop upon the size or extent of the organization or object in question.
An intensive belongings is a bulk property, significant that it is a physical property of a system that does not depend on the system size or the corporeality of material in the arrangement. Examples of intensive properties include temperature, refractive alphabetize, density, and hardness of an object. When a diamond is cut, the pieces maintain their intrinsic hardness (until their size reaches a few atoms thick). In dissimilarity, an all-encompassing belongings is additive for independent, non-interacting subsystems. The property is proportional to the amount of material in the system.
Intensive properties: A physical property that will be the same regardless of the amount of matter.
- density: \(\rho=\frac{m}{v}\)
- colour: The pigment or shade
- conductivity: electricity to flow through the substance
- malleability: if a substance can be flattened
- luster: how shiny the substance looks
Extensive Backdrop: A physical property that volition change if the amount of matter changes.
- mass: how much affair in the sample
- volume: How much space the sample takes up
- length: How long the sample is
Concrete Alter
Change in which the matter'due south concrete appearance is contradistinct, but composition remains unchanged.
A physical modify takes place without whatsoever changes in molecular composition. The same chemical element or compound is present before and afterward the change. The same molecule is present through out the changes. Physical changes are related to concrete properties since some measurements require that changes be made. The three main states of affair are: Solid, Liquid, Gas
- Solid is distinguished by a fixed structure. Its shape and volume do non change. In a solid, atoms are tightly packed together in a stock-still arrangement.
- Liquid is distinguished past its malleable shape (is able to form into the shape of its container), but constant volume. In a liquid, atoms are close together but not in a fixed organisation.
- Gas is made up of atoms that are carve up. However, dissimilar solid & liquid, a gas has no stock-still shape and volume.
Example \(\PageIndex{ane}\): Physical Change
When liquid water (\(H_2O\)) freezes into a solid state (water ice), it appears changed; However, this change is only concrete as the the composition of the constituent molecules is the aforementioned: xi.19% hydrogen and 88.81% oxygen by mass.

Effigy \(\PageIndex{2}\): Physical Change: Ice Melting is a physical change. from Wikipedia.
Chemic Properties and Changes
Chemical properties of thing describes its "potential" to undergo some chemical change or reaction by virtue of its composition. What elements, electrons, and bonding are present to give the potential for chemical change. It is quite difficult to define a chemic property without using the word "change". Eventually you should exist able to await at the formula of a chemical compound and state some chemical property. At this fourth dimension this is very difficult to do and you are non expected to be able to do information technology. For instance hydrogen has the potential to ignite and explode given the right conditions. This is a chemical property. Metals in general have they chemical property of reacting with an acid. Zinc reacts with hydrochloric acid to produce hydrogen gas. This is a chemical property.
Chemic change results in i or more substances of entirely different limerick from the original substances. The elements and/or compounds at the starting time of the reaction are rearranged into new product compounds or elements. A Chemical CHANGE alters the composition of the original matter. Unlike elements or compounds are present at the end of the chemical alter. The atoms in compounds are rearranged to make new and different compounds.
Instance \(\PageIndex{1}\): Corrosion of Metals
Corrosion is the unwanted oxidation of metals resulting in metal oxides.
\[2 Mg + O_2 \rightarrow ii MgO\]

Problems
The following questions are multiple selection.
1. Milk turns sour. This is a ________________
- Chemic Change
- Physical Alter
- Chemic Belongings
- Physical Belongings
- None of the above
2. HCl beingness a potent acrid is a __________, Wood sawed in two is ___________
- Chemical Change, Physical Modify
- Physical Change, Chemical Change
- Chemical Belongings, Physical Change
- Concrete Holding, Chemical Change
- None of the above
three. CuSO4 is dissolved in water
- Chemical Alter
- Physical Change
- Chemical Property
- Physical Property
- None of the above
4. Aluminum Phosphate has a density of 2.566 g/cm3
- Chemical Modify
- Physical Change
- Chemical Property
- Physical Holding
- None of the above
v. Which of the following are examples of matter?
- A Canis familiaris
- Carbon Dioxide
- Ice Cubes
- copper (Ii) nitrate
- A Moving Car
6. The formation of gas bubbles is a sign of what type of alter?
7. True or Simulated: Staff of life rising is a concrete belongings. 8. True or False: Dicing potatoes is a physical alter. ix. Is sunlight matter? 10. The mass of lead is a _____________property.
Solutions
- chemical change
- chemical property, physical change
- physical change
- physical belongings
- All of the in a higher place
- chemical
- Faux
- True
- No
- physical property
References
- Petrucci, Bissonnette, Herring, Madura. General Chemistry: Principles and Modern Applications. Tenth ed. Upper Saddle River, NJ 07458: Pearson Education Inc., 2011.
- Cracolice, Peters. Basics of introductory Chemistry An agile Learning Approach. Second ed. Belmont, CA 94001:Brooks/Cole, 2007.
Contributors and Attributions
- Samantha Ma (UC Davis)
Source: https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Supplemental_Modules_and_Websites_%28Inorganic_Chemistry%29/Chemical_Reactions/Properties_of_Matter
Posted by: mcclendonantaistry.blogspot.com

0 Response to "How Does Chemical Change Affect The Composition Of Matter"
Post a Comment